News & Events

Purification of therapeutic bacteriophages
Endotoxin (LPS) removal
Our phage therapy department investigated three chromatography systems featuring a flowthrough mode to compare performances when carrying out purification of therapeutic bacteriophages through endotoxin removal (also known as LPS removal). Our process development team hereby strengthens their knowledge of GMP-compatible processes for application to various industrial scales.
The emergence of antibiotic-resistant bacteria is one of the most prominent concerns of the World Health Organization (WHO). Administering therapeutic bacteriophages is a potential solution to mitigate the threat posed by antibiotic resistance. Implementing new systems for the manufacture and purification of therapeutic bacteriophages in compliance with Good Manufacturing Practices (GMP) will prove essential if we are to use these viruses as medicinal products.
As with viruses produced in mammalian cells, lysis cycles follow a production of bacteriophages in bacteria. Bacterial lysates contain bacteriophages and various impurities requiring removal prior to administration to allow for safe conditions, devoid of undesirable side effects such as immune response or septic shock. Bacterial lysates are subject to purification steps to ensure that optimal yields are reached while maintaining quality and potency of bacteriophages, in particular through residual contaminant removal. Specific attention should be devoted to endotoxins (EU for Endotoxin Unit), generated from gram-negative bacteria, whose concentration should not exceed 5 EU/Kg/h for parenteral medicinal products. Endotoxin removal is often challenging as yield loss tends to occur when separating bacteriophages from endotoxins.
The Process Development department of Clean Cells has recently worked at identifying an efficient method to remove LPS from bacteriophage bulks. The main objectives to be achieved for the purification of therapeutic bacteriophages were as follows:
- Efficiency and performance
- Simple and quick setup
- Applicability to various industrial scales and compatibility to GMP
In this work, three systems were tested, two in chromatography flowthrough mode and one in chromatography positive mode. An efficient purification method was then developed using one of the best systems with the final goal of achieving the pre-defined criteria. This method allows for a 4 log reduction of EU, achieving concentration below 1 EU/109 PFU while maintaining a very high yield, close to 100%.
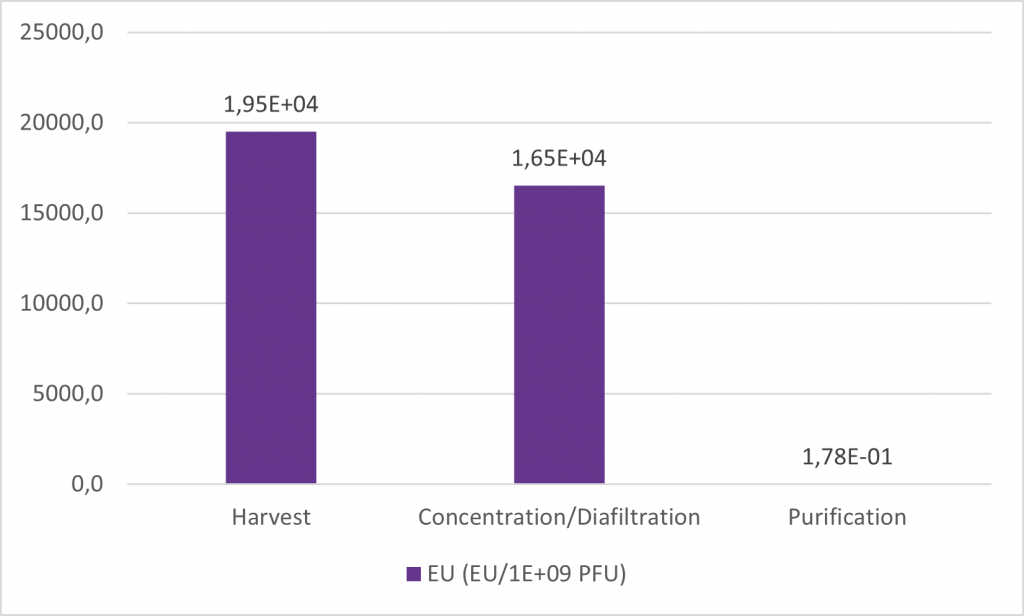
Detailed information about this comparison study is available here: