Just after the discovery in 1917 by Felix d’Hérelle of bacteriophages, the natural-born killers of bacteria, their use have been rapidly recognized by a large number of scientists as a possible way to cure bacterial infections. In 1919, a first patient was cure for diphtheria using bacteriophages. Phagotherapy was born! Phages infect specifically bacteria letting safe patient’s cells. More explanations in picture
George Eliava, a georgian microbiologist, founds in 1923 at Tbilissi, Georgia, a institute of virology where he studies from 1930 bacteriophages and the application of phagotherapy for the Soviet Union. From that time, the use of bacteriophages to cure remains a common practice in Georgia that is nowadays subjected to important health tourism.
Since the advent of antibiotics in 1940, phagotherapy falls utterly in disuse. Coming back on stage in Europe during the seventies, phagotherapy appears today as a promising alternative way to fight multidrug-resistant bacteria that are notably responsible for nosocomial infections. The challenge of this therapy is to get it over with multidrug-resistant bacteria in turning phages into a new drug. Indeed, since a couple of years, the resistance of antibiotic and the lack of development towards new treatments to cure bacterial infections are responsible for a growing number of therapeutic deadlocks. Today, it is estimated that more than 12,000 deaths are due to antibiotics resistances.
In this health context, Pherecydès Pharma has call out Clean Cells for the manufacturing of phages cocktail batches dedicated to the treatment of badly burned patients and Clean Cells has came forward and took up this challenge. It’s taking on the manufacturing and the quality control of these phages for a therapeutic use within the frame of the European collaborative project Phagoburn that Clean Cells initiates phagotherapy. Today, a clinical trial is currently being carried out (to treat about a hundred patients suffering from skin burns with a definite risk of surinfection with bacteria).
Also, thanks to the authorization to produce experimental drugs in compliance with good manufacturing practice (GMP), Clean Cells, as an authorized pharmaceutical facility, is now in position to offer its customers a wide panel of services for drug manufacturing as well as for biosafety throughout the whole manufacturing process, as depicted in the following scheme:
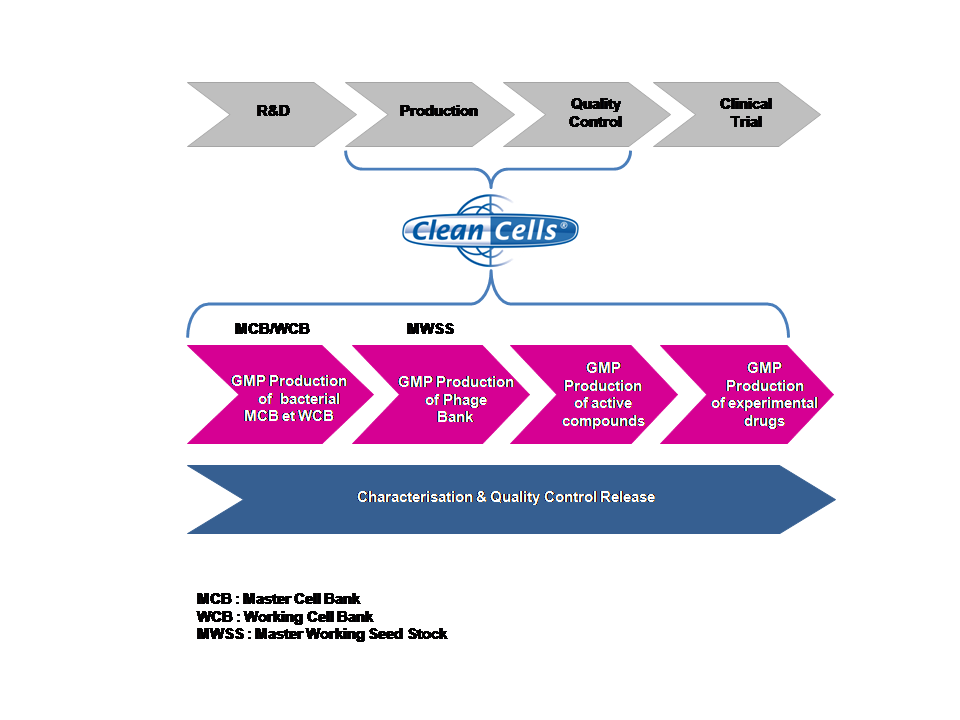
To learn more about our production and quality control capabilities , please contact Audrey Larrieu, head of production, at 02 51 09 27 40 or by email: alarrieu@clean-cells.com
Replay the Laurent Bretaudeau’s lecture, R&D director at Clean Cells, at the EMA Workshop on the therapeutic use of bacteriophages (London, 8 June 2015)