Mycoplasma: Detecting the invisible threat!
Understanding Mycoplasma: A persistent contamination risk
Mycoplasma are small, cell wall-free bacteria belonging to the Mollicutes class. They are notorious contaminants in cell culture due to their ability to:
- Difficult to identify: They evade standard microscopic detection,
- Biological interferences: They alter cellular metabolism and functions,
- Persistent survival: As a parasitic organisms, they exploit host cells for nutrients and growthients and growth.
Why is Mycoplasma testing critical?
- Human and animal impact: Mycoplasma can infect humans, animals, and plants, causing respiratory and genital infections
- Bioproduction risks: Undetected contamination can compromise batch integrity and patient safety
- Regulatory guidelines: Global guidelines (EP 2.6.7, USP <63>) mandate rigorous mycoplasma testing during drug development and manufacturing.
Clean Cells offers comprehensive mycoplasma detection services, in line with GMP and non-GMP standards, ensuring the highest level of biosafety and regulatory compliance.
Comprehensive Mycoplasma testing services
We integrate gold-standard compendial methods with cutting-edge rapid technologies, offering flexibility, reliability, and regulatory compliance for biopharma, vaccines, and cell & gene therapy developers.
Classical Compendial Culture Methods (EP 2.6.7, USP <63>)
Direct culture method
- Incubation under microaerophilic conditions for 28 days
- Relies on specific liquid and solid media to support the growth of key mycoplasma strains
(ie M. pneumoniae and M. orale) - Requires high sample volume (~35 mL)

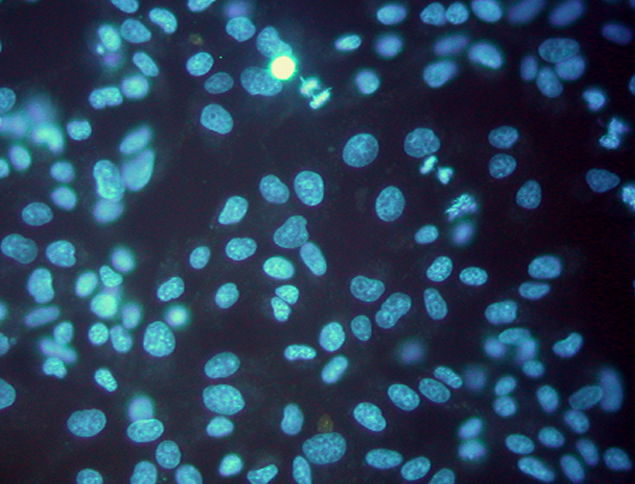
Indirect culture method (Fluorescence staining)
- Amplification using Vero cell, followed by Hoechst DNA staining for Mycoplasma visualization
- Shorter incubation time than Direct Culture (7 days)
- Needs two different mycoplasma strains
(M. Hyorhinis and M. orale) - Requires 2 to 4 mL of sample
Advanced molecular technologies for faster results
In-house PCR
with or without enrichment
(GMP and ICHQ2R2 compliant)
- Detect all relevant mycoplasma strains
- Validated for GMP and regulatory compliance per EP 2.6.7 and EP 2.6.21
- Rapid turnaround time: 24 to 48 hours
- Requires only 1 mL
- 21 CFR Part 11 compliant, ensuring data integrity and traceability
BIOFIRE®
- Ultra-fast results in just few hours: ideal for fast release & short-life cell therapy products
- Requires low sample volume – Perfect for cell & gene therapy
- Optimized for GMP environments: Fully validated and compliant with EP 2.6.7, USP <63>
- Can be implemented in-house or outsourced
- Clean Cells supports method validation & regulatory approval
- 21 CFR Part 11 compliant, ensuring data integrity and traceability
Learn more about our partnership with bioMerieux!
Why choose Clean Cells for Mycoplasma Testing?
- 20+ years of expertise in mycoplasma detection and regulatory compliance
- Comprehensive testing portfolio: Culture-based methods, PCR, and BIOFIRE® rapid PCR
- GMP-compliant laboratories: ensuring regulatory adherence and data reliability
- Tailored solutions for biopharma, vaccine manufacturers, and cell & gene therapy developers
- Expert validation services: supporting BIOFIRE® implementation in your laboratory
Clean Cells, a Pioneer in Mycoplasma Detection for Biologics
Since 2000, Clean Cells has been commited to the safety of biological products, in part by preventing mycoplasma contamination. These insidious micro-organisms represent a major risk to cell culture systems, biopharmaceutical production and patient safety, hence the importance of robust, compliant detection methods.
Contact us to discuss your mycoplasma testing needs and ensure the highest level of biosafety for your biologics!


