News & Events

Patent for potency assay evaluating cytolytic activity
Potency evaluation of cytolytic activity: cutting-edge innovation for your product
Our assay development team at Clean Cells continuously works on innovative methods for the potency evaluation of biologics. Today, the patent for the method used to assess cytolytic activity in biologics is extended to Canada, thus illustrating the growth of Clean Cells and its inherent assay development expertise. Suitable for ADCC/CDC studies within the evaluation of monoclonal antibodies, or to evaluate CAR-T cells’ specific killing capacity, the assay displays increased sensitivity and allows for precise measurement of cell death induced by your biological product.
The method
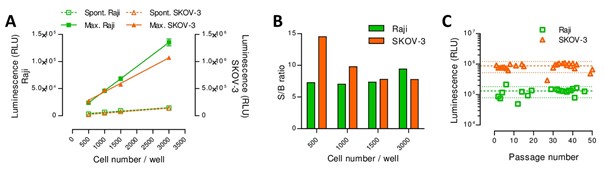
This innovative potency assay is based on luminescence, thus circumventing the use of radioactivity, and streamlining the assay integration (requires luminometers only) and execution.
The method uses a reporter gene expressed in an engineered cell line: it allows for increased specificity and standardization and answers regulatory requirements relating to the potency evaluation of cytolytic activity.
Overall, the assay displays a linear signal and shows increased sensitivity compared to comparable methods.
Patents and expansion of the Clean Biologics group
A patent has initially been granted in Sept 2016 in France, where the method was first developed. This patent was then extended to the European area in December of 2020.
Today, our patent filed in Canada has been delivered in August 2021 and illustrates both the advances made by Clean Cells in the field of assay development and the expansion of the Clean Biologics group, which acquired Canadian company BioDextris in May 2021.
Integration in quality control service offer
This potency assay is part of a quality control service offer strengthened by Clean Cells for more than 20 years.
Clean Cells, a GMP-certified company, offers 250+ tests to characterize all types of biologics based on potency, biosafety, identity, and purity criteria.
Please reach out to our team to discuss quality control testing needs and to tailor the most suited testing plan according to your product and development stage.