
The folder : BSL3 GMP manufacturing suite - Naobios
Our team at Naobios has qualified a highly-secured BSL3 GMP manufacturing suite in our multipurpose facility to accommodate challenging GMP production projects involving viral vaccines, viral vectors and oncolytic viruses.
Our news :

The importance of technical oversight (CMC) in drug development
Technical oversight brought by a Chemistry, Manufacturing and Controls Subject Matter Expert (CMC SME) is crucial to ensure acceptance with regulatory bodies and patient safety at every stage of GMP manufacturing and in association with CDMO services provided by the Clean Biologics group.
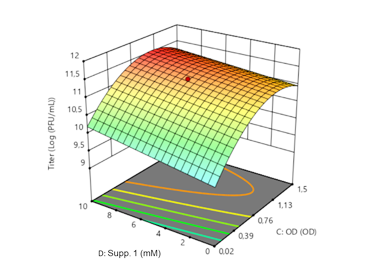
Upstram process optimization of bacteriophage production using Design of Experiment
Clean Cells’ process development department has worked on implementing a robust and flexible Design of Experiment approach for upstream process optimization leading to higher titers and the characterization of a design space.
