The quarterly scientific journal

15/11/2021
BSL3 GMP manufacturing suite – Naobios
Naobios is a GMP-certified company with a high-quality and long-term experience in manufacturing of preclinical and clinical lots of viral vaccines (live or inactivated), viral vectors and oncolytic viruses. Naobios is a GMP establishment certified by French authorities (ANSM).
Extensive manufacturing suites and capabilities
In terms of manufacturing capabilities, Naobios provides:
- A multi-purpose facility with production by campaign
- Operations defined for the manufacturing of:
- Cell banks
- Viral seed banks
- Drug Substance: inactivated or live viral vaccines, viral vectors
- Process validation: inactivation process, aseptic process, cleaning
- Maximum production working volumes: 200L
- Adapted suites for GMO, BSL2 and BSL3 pathogens
A highly-secured BSL3 GMP manufacturing suite
Our BSL3 GMP manufacturing suite is a GMP unit of 110m2 (1184ft²) featuring Class D and Class C clean rooms fulfilling the requirements to handle organisms belonging to Risk Group 3:
- All safety and environmental requirements
- Solid waste decontamination by a dedicated autoclave while liquid waste undergoes station-mediated continuous decontamination.
- Critical environmental parameters are monitored 24/7 by an Environmental Monitoring System (EMS).
- Operations are strictly segregated and flows of people, material and waste have been designed per GMP requirements.
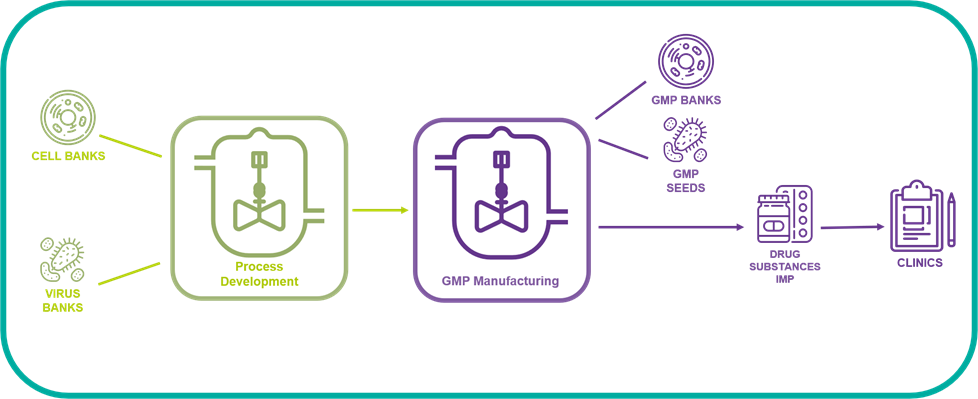
The BSL3 GMP manufacturing suite may accommodate various stages of bioproduction:
#STEP 1: Virus stock / Cell bank production process:
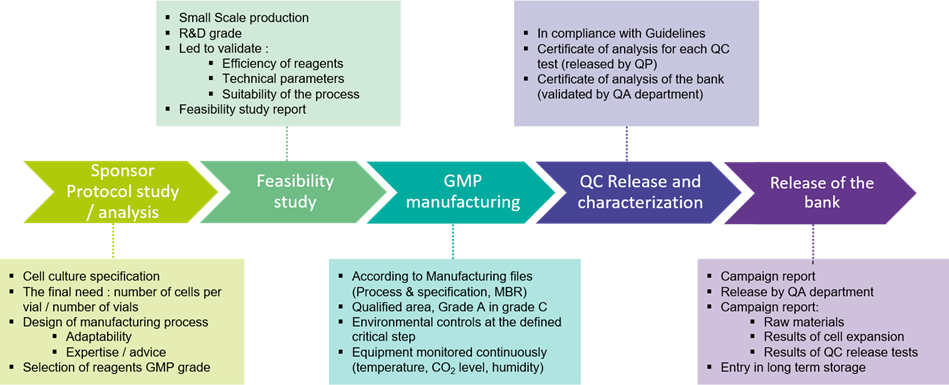
#STEP 2: Bioproduction